

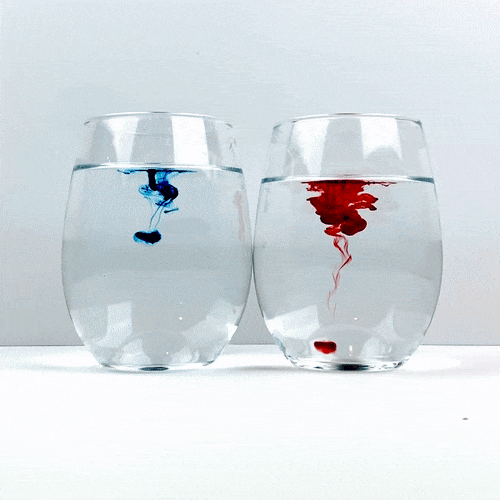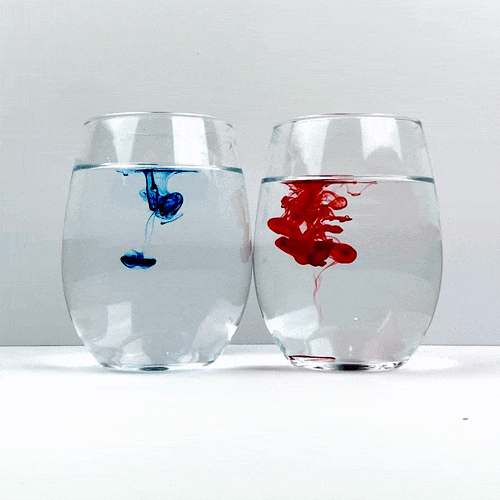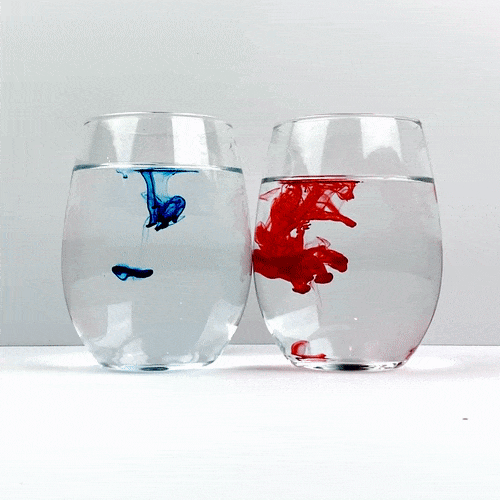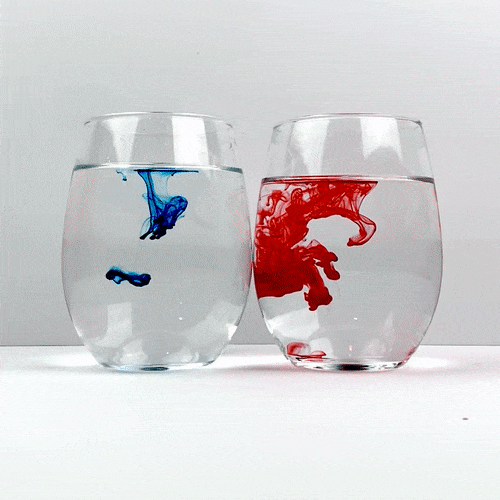
Did you know that particles are always in motion? It's true, the crazy things never sit still! All types of particles, whether they're subatomic particles (electrons, protons, and neutrons), atoms, or molecules, are constantly moving around, bumping into one another, or vibrating. In fact, scientists only know of one possible, but never observed, situation where particles sit still: at a temperature known as absolute zero, a mind-bogglingly chilly -273.15°C, where particles have no heat energy at all!
You might've just guessed, then, that the motion of particles is somehow related to how much heat energy they have. And it's true! As particles are heated up, they start to move faster and faster, and bump into each other with more and more energy. If you've ever tried to stir sugar into cold tea, you've already witnessed the effect that temperature has on molecular motion. The hotter your tea is, the more water molecules are going to bump into the molecules of sugar, dissolving it faster. In this experiment, we're going to take this idea and go one step further and try to visualize molecular motion in full effect.

Fill each of the drinking glasses to about the halfway point, one with hot water and the other with cold water. Alternatively, if you like, you can try this experiment with more glasses and a range of different temperatures.

Trying not to give either of the glasses a head start, drop a single drop of food coloring into each glass. Be careful not to accidentally move one of the glasses and remember not to stir!
If you're having trouble getting the food coloring to drop into each glass at the same time by yourself, try asking a labmate to help you out. Sit next to each other so you can see both glasses at once, and on the count of monkey (one... two... three... monkey!), you can each drop one drop of food coloring into the glass in front of you.

Carefully observe how the food coloring spreads throughout the water. Which one is spreading the quickest?
You must be logged in to comment.